Patient Registries
Registries are necessary in all types of research and for epidemiological surveillance. As rare diseases are rare and patient numbers are therefore often limited, sharing of data is absolutely crucial.
VASCERN aims to build rare vascular disease registries and to make already existing registries accessible by using the FAIR principles.
The FAIR data principles are a set of principles used to make data findable, accessible, interoperable and reusable (FAIR). They also provide the guidelines for good scientific data management and stewardship in order to maximize the use of valuable research data by the scientific community. In order to make data FAIR, the first step is to make data findable, by assigning a globally unique and persistent identifier to data/metadata. Next, one must describe data/metadata using ontologies and vocabularies and generating machine-readable data. A semantic model, that describes the relations between the concepts, can be made in order to create this machine-readable data. The accessibility of the data must be also well defined. Metadata can be made available on a FAIR Data Point, making it findable online. Finally, data must be reusable, meaning that it can be reproduced and reused, so it must be richly described and provide clear usage licenses and accurate information on its provenance.
Our Patient Registry Working Group is responsible for this workpackage and the VASCERN Registry Technical Team meets every 3 weeks in order to discuss the technical aspects of the individual RDWG registry databases, their FAIRification, and their evolution.
For more information on the Patient Registry working group and its members click here
To learn more about the FAIR principles, read the following publication on FAIR here
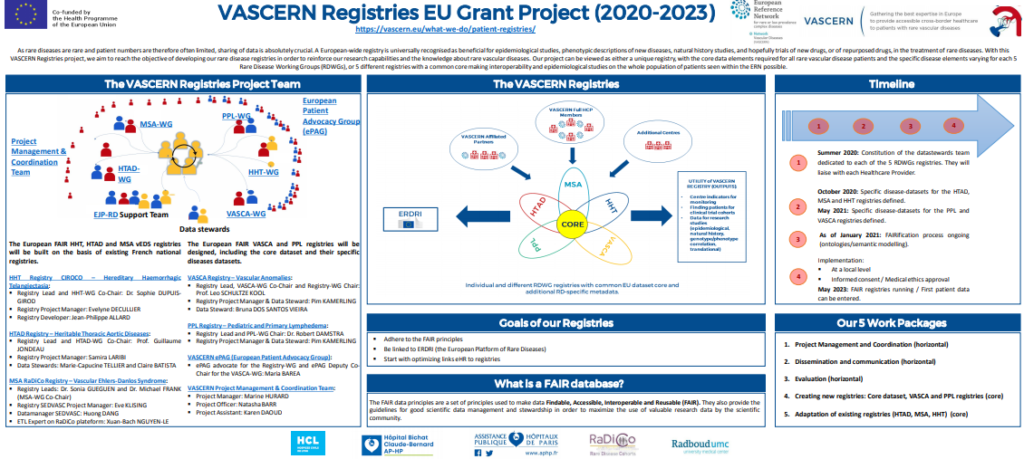
VASCERN Registries EU Grant Project (2020-2023)
The VASCERN Registries project aims to reach the objective of developing our rare disease registries in order to reinforce our research capabilities and the knowledge about rare vascular diseases. Our project can be viewed as either a unique registry, with the core data elements required for all rare vascular disease patients and the specific disease elements varying for each 5 Rare Disease Working Groups (RDWGs), or 5 different registries with a common core making interoperability and epidemiological studies on the whole population of patients seen within the ERN possible.
To learn more abou this project take a look at our informational poster below:
[cq_vc_accordion][/cq_vc_accordion]
Latest Registry Updates by RDWG
HHT Registry CIROCO
- The database is accessible on the internet (it was agreed within the HHT WG that only the minimum dataset would be filled in for the moment at the European level).
- The dictionary of variables from the CIROCO database has been translated from French into English.
- They are currently working on the FAIR data point with the VASCERN data stewards.
- Within HHT HCP Hospices Civils de Lyon, they are working on the authorizations with the CNIL (French data protection authority) and the contracts to be transmitted to each European center to be signed by their clinical research department and the legal department.
HTAD Registry
- The programme has been implemented (CleanWeb) and is in production with the minimum dataset and the HTAD specific disease elements defined
- HTAD HCP APHP Hopital Bichat, is already entering patient data
- Accounts have been created for other HTAD centers in Europe and now they are awaiting the signature of legal documentation from these centers in order to start entering patient data
- The database has been declared on ERDRIdor (European Directory of Registries)
MSA Registry – RaDiCo vEDS
- Implementation of the program (REDCap): database already available on the RADICO local server and accessible through the web
- The registry address has been exported to the ERDRIdor
- Encoding of the dataset (Orphanet, HPO, OMIM) is done
- Definition of the dataset: minimum dataset and vascular Ehlers-Danlos syndrome (vEDS) specific data elements is completed
- Exporting the registry metadata on the ERDRImdr is completed
Coming soon!!!
PPL Registry
- PPL registry (using IT platform Castor) is technically ready to start registering patients
- It has been registered at ERDRI.dor and ERDRI.mdr.
- PPL HCP Nij Smellinghe hospital has signed the contract with Castor and the installation of Castor is now taking place.
- PPL HCP Helsinki University Hospital almost ready to register patients: has signed permit for using the hospital data and soon to sign contract with Castor
- Disease specific elements are defined and are at EJP-RD for selecting and modeling
- The German PPL HCP, Földiklinik, has also signed the contract with Castor.
VASCA Registry
- VASCA registry is technically ready to accept patients. With the help of our VASCERN data steward, VASCA HCP Radboud University Medical Center had started registering patients with EUPID (pseudonymisation tool) but as EUPID is no longer used because of security issues these patients have been removed. There is a new pseudonymisation tool being considered as a replacement called SPIDER (Secure Privacy-preserving Identity management in Distributed Environments for Research). This tool is still under development and discussed within the ERN’s.
- It has been registered at ERDRI.dor and ERDRI.mdr.
- VASCA HCP Helsinki University Hospital almost ready to register patients: has signed permit for using the hospital data and soon to sign contract with Castor
- Disease specific elements are defined and are at EJP-RD for selecting and modeling



