The VASCA Working Group, built upon Multidisciplinary Centres of Excellence for Vascular Anomalies
*NEW* First edition of the VASCA Magazine available here 
The Vascular Anomalies Working Group (VASCA-WG) is one of the five Rare Disease Working Groups (RDWGs) of VASCERN that specialize in a particular type/group of rare vascular disease(s).
The VASCA-WG is chaired by Professors Miikka VIKKULA and Laurence BOON, from the Centre for Vascular Anomalies at Cliniques Universitaires Saint-Luc in Brussels, Belgium, and Co-Chaired by Professor Leo SCHULTZE KOOL from the Expert Center for Hemangioma and Vascular Anomalies (HECOVAN) at Radboudumc in Nijmegen, Netherlands.
The VASCA-WG cooperates with the European Patient Advocacy Group (ePAG), whose representatives participating in the VASCA-WG are ePAG Co-Chair for VASCA, Caroline VAN DEN BOSCH (HEVAS) and ePAG Deputy Co-Chair Maria BAREA (VASCAPA).
The VASCA-WG members are also active in the International Society of the Study of Vascular Anomalies (ISSVA), which is based in the USA. VASCA-WG members are on the ISSVA scientific committee and the ISSVA Board. Thanks to this, they are part of a major international network with clinicians within the EU and the rest of the world and have been implicated in the identification of most of the genes involved in vascular anomalies and are active in research testing new targeted therapies.
The Center for Vascular Anomalies at Cliniques universitaires Saint-Luc in Brussels Belgium (and other HCP members of the VASCA-WG) takes care of a whole variety of pathologies within the field of Vascular anomalies (see: Wassef et al., Revised classification of Vascular Anomalies, 2015 Pediatrics, and ISSVA web-site).
The Rare Diseases included in this ERN Diseases Working Group are (non exhaustive list):
Bases for the estimated incidence and prevalence numbers: 500 Million inhabitants within European union with about 6 million newborns annually.
| sub-thematic areas of expertise | Rare or complex disease(s) or condition(s) or highly specialized interventions | Code/ICD/ Orphacode / Group of Codes | Incidence (Number of cases / year) (in the EU) | Prevalence (in the EU) |
|---|---|---|---|---|
| VASCA | Rare arteriovenous malformation | ORPHA211266 | 120 | 10000 |
| VASCA | Blue Rubber Bleb Nevus syndrome | ORPHA1059 | 6 | 500 |
| VASCA | Capillary malformation-arteriovenous malformation | ORPHA137667 | 120 | 10000 |
| VASCA | Cerebral arteriovenous malformation | ORPHA46724 | ||
| VASCA | CLAPO syndrome | ORPHA168984 | ||
| VASCA | CLOVES syndrome | ORPHA140944 | 60 | 5000 |
| VASCA | Cutis Marmorata Telangiectatica Congenita | ORPHA1556 | 12 | 1000 |
| VASCA | Diffuse neonatal hemangiomatosis | ORPHA2123 | ||
| VASCA | Facial arteriovenous malformation | ORPHA156230 | ||
| VASCA | Familial cerebral cavernous malformation | ORPHA221061 | 600 | 50000 |
| VASCA | Diffuse lymphatic anomaly | ORPHA141209 | ||
| VASCA | Glomuvenous malformation | ORPHA83454 | 30 | 2500 |
| VASCA | Gorham-Stout syndrome | ORPHA73 | 6 | 500 |
| VASCA | Infantile hemangioma of rare localization | ORPHA210589 | ||
| VASCA | Kaposiform hemangioendothelioma | ORPHA2122 | ||
| VASCA | Klippel-Trénaunay-Weber syndrome | ORPHA2346 | 60 | 5000 |
| VASCA | LUMBAR association | ORPHA83628 | ||
| VASCA | Rare lymphatic malformation | ORPHA2415 | 600 | 50000 |
| VASCA | Megalencephaly-capillary malformation-polymicrogyria syndrome | ORPHA60040 | 12 | 1000 |
| VASCA | Macrocystic lymphatic malformation | ORPHA79489 | ||
| VASCA | Maffucci syndrome | ORPHA163634 | 6 | 500 |
| VASCA | Microcystic lymphatic malformation | ORPHA79490 | ||
| VASCA | Mixed cystic lymphatic malformation | ORPHA458792 | ||
| VASCA | Mucocutaneous venous malformation | ORPHA2451 | ||
| VASCA | Non-involuting congenital hemangioma | ORPHA141179 | 20 | 500 |
| VASCA | Primary intralymphatic angioendothelioma | ORPHA458768 | ||
| VASCA | Parkes-Weber syndrome | ORPHA90307 | 30 | 2500 |
| VASCA | Partially-involuting congenital hemangioma | ORPHA458785 | ||
| VASCA | PHACE syndrome | ORPHA42775 | ||
| VASCA | Proteus syndrome | ORPHA744 | 6 | 500 |
| VASCA | PTEN hamartoma tumor syndrome | ORPHA306498 | 30 | 2500 |
| VASCA | Pulmonary arteriovenous malformation | ORPHA2038 | ||
| VASCA | Rapidly involuting congenital hemangioma | ORPHA141184 | 60 | 5000 |
| VASCA | Rare capillary malformation | ORPHA211247 | 60 | 5000 |
| VASCA | SACRAL association | ORPHA2125 | ||
| VASCA | Spindle cell hemangioma | ORPHA210584 | ||
| VASCA | Sturge-Weber syndrome | ORPHA3205 | 120 | 10000 |
| VASCA | Tufted angioma | ORPHA1063 | 3 | 250 |
| VASCA | Rare venous malformation | ORPHA211252 | 1200 | 100000 |
| VASCA | Verrucous hemangioma | ORPHA464318 |
In this ERN, there is a specific WG for Pediatric and Primary Lymphedemas.
Hereditary hemorrhagic telangiectasia (HHT, orphanet: 774, ICD‐10: I78.0) is also a vascular anomaly, but for historical reasons specialised centers have been build up for that single diseases on the past. HHT is included as a separate WG within the ERN (see HHT WG).

BELGIUM
Cliniques universitaires Saint-Luc, Brussels, Belgium
VASCERN VASCA European Reference Centre, Center for Vascular Anomalies


Chair
Prof. Miikka VIKKULA
Clinical Geneticist

Prof. Laurence M. BOON
Plastic and Reconstructive Surgeon

ePAG Deputy Co-Chair
Maria BAREA

NETHERLANDS
Radboud university medical center, Nijmegen, Netherlands
VASCERN VASCA European Reference Centre, Expert center for Hemangioma and Vascular Anomalies (Hecovan)


Co-Chair
Prof. Leo SCHULTZE KOOL
Interventional Radiologist

Dr. Carine VAN DER VLEUTEN
Dermatologist

ePAG Co-Chair
Caroline VAN DEN BOSCH

FINLAND
Helsinki University Hospital, Helsinki, Finland
VASCERN VASCA European Reference Centre, Department of Pediatric Surgery


FRANCE

DENMARK

GERMANY
Medical Center – University Freiburg, Freiburg, Germany
VASCERN VASCA European Reference Centre, Center of Pediatrics and Adolescent Medicine


Dr. Friedrich KAPP
Pediatric Hematologist/Oncologist

Prof. Jochen RÖßLER
Pediatric Hematologist/Oncologist

IRELAND
Children’s Health Ireland, Dublin, Ireland
VASCERN VASCA European Reference Centre, Dermatology Department


ITALY
Bambino Gesù Children’s Hospital, I.R.C.C.S, Rome, Italy
VASCERN VASCA European Reference Centre, Dermatology Department


LITHUANIA
Vilniaus Universiteto ligoninė Santaros Klinikos, Vilnius, Lithuania
VASCERN VASCA European Reference Centre


SPAIN
Hospital Sant Joan De Déu (SJD Barcelona Children’s Hospital), Barcelona, Spain
VASCERN VASCA European Reference Centre


NORWAY

PORTUGAL
Centro Hospitalar Universitário de São João, Porto, Portugal
VASCERN VASCA European Reference Centre


SWEDEN
Karolinska University Hospital, Stockholm, Sweden
VASCERN VASCA European Reference Centre, Department of Pediatric Surgery

AFFILIATED PARTNERS
There are 2 types of affiliated partners:
- Associated National Centres (mainly healthcare providers) for those ERNs where an EU Member State is not yet represented by a full member in the respective ERN. It establishes a link with one specific ERN.
- A National Coordination Hub which establishes at once a link with more than one Network in which a given Member State is neither represented by a full member nor by an Associated National Centre. National Coordination Hubs may especially represent a useful solution for those Member States with very small populations that need to establish such links with many ERNs at once.
For all documents related to Affiliated Partners see the Board Statement States page here
Associated National Centres

AUSTRIA
Pills of Knowledge (PoK)
Pills of Knowledge (PoK) are the deliverable for VASCERN Work Package 4 on Pills of Knowledge, defined as short single video lessons (of approximately 3-5 minutes long) in which an expert talks about a specific topic that has been selected and validated by the Rare Disease Working Groups (RDWGs).
Link to the playlist for the VASCA-WG on our YouTube Channel is here
Classification of Vascular Anomalies
Created by Prof. Laurence Boon (Plastic Surgeon, Coordinator of the Center for Vascular Anomalies, Cliniques Universitaires St Luc, Brussels, Belgium).
This Pill of Knowledge (PoK) gives a brief overview of the classification of vascular anomalies. It is intended for the medical community who is not familiar with these anomalies as it introduces the main types of vascular anomalies and their characteristics.
Video in English. Subtitles available in 8 European languages (English, Dutch, Finnish, French, German, Italian, Spanish and Swedish).
Diagnostic Approaches for Vascular Anomalies
Created by Dr. Friedrich Kapp (Physician Scientist, University Medical Center, Freiburg, Germany).
This Pill of Knowledge (PoK) talks about the diagnostic approaches for vascular anomalies. The various types of examination and tests involved in reaching an appropriate diagnosis are described. This PoK is a valuable tool for healthcare professionals and patients wanting to learn more about how vascular anomalies are diagnosed.
Video in English. Subtitles available in 8 European languages (English, Dutch, Finnish, French, German, Italian, Spanish and Swedish).
Multi-disciplinary Expertise Teams for Vascular Anomalies
Created by Prof. Leo Schultze Kool (Interventional Radiologist, Radboud University Medical Center, Nijmegen, Netherlands).
This Pill of knowledge (PoK) gives a brief summary of why multi-disciplinary expert teams are so necessary for the management and treatment of vascular anomalies. It is intended for the general public.
Video in English. Subtitles available in 8 European languages (English, Dutch, Finnish, French, German, Italian, Spanish and Swedish).
Management of Vascular Anomalies
Created by Dr. Kristiina Kyrklund (Pediatric Surgeon, Helsinki University Hospital, Helsinki, Finland)
In this Pill of Knowledge (PoK) Dr. Kristiina Kyrklund gives an introduction on the individualized management of vascular anomalies and how the chosen treatment is based on the characteristic symptoms and location of the anomaly. Suitable for both healthcare professsionals and patients.
Video in English. Subtitles available in 8 European languages (English, Dutch, Finnish, French, German, Italian, Spanish and Swedish).
The lymphatic system & lymphatic malformations (Lymfestelsel en lymfatische malformaties) – video in Dutch
This video, produced by the patient organisation HEVAS and validated by the VASCA-WG, talks about the lymphatic system and lymphatic malformations. This video has been made for patients and introduces the patient organisation HEVAS and their work.
View this video here
This video is now available in English (see below).
Treatments for lymphatic malformations (Behandelingen voor lymfatische malformaties) – video in Dutch
This video, produced by the patient organisation HEVAS and validated by the VASCA-WG, describes the treatments currently available for lymphatic malformations in a clear and understandable manner suited for patients.
View this video here
This video is now available in English (see below).
The lymphatic system & lymphatic malformations
This PoK video (produced by the patient organisation HEVAS and revised and validated by the VASCA-WG) gives an introduction on the lymphatic system and lymphatic malformations. It is intended for patients and the general public and uses simple language to explain the various medical terms discussed.
Video in English. Subtitles are currently available in 7 European languages (English, Dutch, French, German, Italian, Spanish and Swedish).
View this video here
Treatment of lymphatic malformations
This PoK video (produced by the patient organisation HEVAS and revised and validated by the VASCA-WG) presents the various treatment options for lymphatic malformations. It is intended for patients and the general public and uses simple language to explain the various medical terms and procedures discussed.
Video in English. Subtitles are currently available in 7 European languages (English, Dutch, French, German, Italian, Spanish and Swedish).
View this video here
Klippel-Trenaunay syndrome (KTS)
This PoK video, produced by the patient organisation HEVAS and validated by the VASCA-WG, gives an introduction to Klippel-Trenaunay syndrome (KTS), a congenital vascular bone syndrome. It is intended for patients and the general public and uses simple language to explain the clinical characteristics of this rare disease.
Video in English. Subtitles are currently available in 7 European languages (English, Dutch, French, German, Italian, Spanish and Swedish).
View this video here
The PIK3CA gene and related vascular malformations
This short PoK video explains how an error in the PIK3CA gene can be the cause of congenital vascular malformations, such as lymphatic and venous malformations, which can be isolated or occur in overgrowth syndromes. Diagnosis and treatment are also briefly covered.
It is intended for patients and the general public and uses simple language to introduce PIK3CA related vascular abnormalities, which includes the complex group of disorders known as PROS (PIK3CA-Related Overgrowth Spectrum).
Created by the patient organisation HEVAS (the Dutch Patient organisation for hemangioma and vascular malformations), this PoK was then revised and validated by VASCERN’s Vascular Anomalies Working Group (VASCA-WG).
Video in English with subtitles in English.
View this video here.
Webinars
VASCERN Webinar: Classification of vascular anomalies
This webinar consists of a scientific presentation followed by a Q&A session.
Featuring Vascular Anomalies Working Group (VASCA-WG) members: Prof. Miikka Vikkula, Prof. Emir Haxhija and Dr. Paolo Gasparella.
Video in English.
VASCERN Webinar: Diagnostic and Management Pathway for Severe and/or Rare Infantile Hemangiomas.
This webinar consists of a scientific presentation followed by a Q&A session.
Featuring Vascular Anomalies Working Group (VASCA-WG) member: Prof Andrea Diociaiuti.
Video in English.
VASCERN Webinar: Diagnostic and Management Pathway for Venous Malformations.
This webinar consists of a scientific presentation followed by a Q&A session.
Featuring Vascular Anomalies Working Group (VASCA-WG) member: Prof. Laurence Boon.
Video in English.
VASCERN Webinar: Diagnostic and Management Pathway for Lymphatic Malformations.
This webinar consists of a scientific presentation followed by a Q&A session.
Featuring Vascular Anomalies Working Group (VASCA-WG) member: Dr Nader Ghaffarpour.
Video in English.
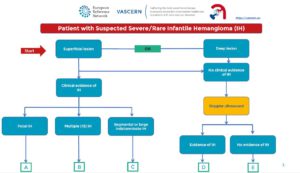
Patient Pathways aim to improve the care and management of patients with a rare disease. They include the “red flags” that may lead to the suspicion of the disease, how to reach a definite diagnosis and the management and follow-up recommendations. They are a very important tool used in defining the best patient care and will be further validated and updated when needed.
The first Patient Pathway by the VASCA-WG on Severe/Rare Infantile Hemangioma (issued 20/03/2019) can be found here
![]() German translation here
German translation here
![]() Italian translation here
Italian translation here
![]() Spanish translation here
Spanish translation here
![]() Swedish translation here
Swedish translation here
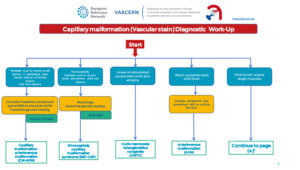
The Capillary Malformation Patient Pathway (issued 29/04/2020) can be found here
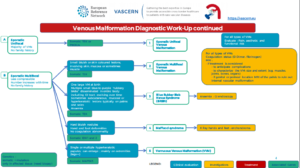
The Venous Malformation Patient Pathway (issued 29/04/2020) can be found here
The Lymphatic Malformation Patient Pathway (issued 29/04/2020) can be found here
- Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, Burrows P, Frieden IJ, Garzon MC, Lopez-Gutierrez JC, Lord DJ, Mitchel S, Powell J, Prendiville J, Vikkula M; ISSVA Board and Scientific Committee. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics. 2015 Jul;136(1):e203-14. doi: 10.1542/peds.2014-3673. Epub 2015 Jun 8. Review.
- On the ISSVA web-site, the classification is available with more detailed genetic data: here
- Stillo et al. Vascular Anomalies Guidelines by the Italian Society for the Study of Vascular Anomalies (SISAV). Angiology, volume 34. April 2015, Suppl 1 to issue No. 2. (These guidelines will be discussed within the VASCA-WG for further development so as to establish first European guidelines).
- A. Elajmi, P. Clapuyt, F. Hammer, A.-C. Bataille, B. Lengele, L.M. Boon. Prise en charge des anomalies vasculaires chez l’enfant. Management of vascular anomalies in children. Annales de chirurgie plastique esthétique (2016). (These guidelines will be discussed within the VASCA-WG for further development so as to establish first European guidelines).
The VASCA-WG will also be working in tight collaboration with ISSVA, which is setting up working groups for establishment of best practice guidelines for various vascular anomalies.
VASCERN Vascular Anomalies (VASCA) Working Group COVID-19 Statement (March 20, 2020)– initiated by the patient advocacy groups in Europe and the United States and their medical advisory teams and approved by the VASCA WG.
This statement can equally be found in:
French: Information COVID-19 pour les patients porteurs d’ Anomalies Vasculaires et/ou Lymphatiques
The VASCA-WG is currently working on creating other expert consensus statement documents on various rare vascular anomalies.
The VASCA-WG is currently working on defining their clinical outcome measures. They are involved in/actively following the ongoing OVAMA (Outcome Measures for Vascular Malformations) project.
The VASCA-WG is currently working on their Do’s and Don’ts Factsheets.
The VASCA Registry project consists of the creation of a FAIR registry dedicated to the diseases covered by the VASCA-WG. It has been designed to include the European core dataset as well as set of data specific to the rare diseases of VASCA.
More information on the Registry WG page here.
The VASCA-WG equally endorses the SECURE-VA registry, which aims to report the COVID-19 cases in Vascular Anomalies (VA) patients globally, in both children and adults.
Multicentre clinical trials:
The VASCA-WG currently has one clinical trial underway: The VASE (Vascular Anomaly-Sirolimus Europe) is phase III multicentric study evaluating the efficacy and safety of sirolimus in Vascular Anomalies that are refractory to standard care.
Current Research Studies
- GLA/GSD: Identification of causative genetic mutations. Brussels and Freiburg HCPs involved (started 2016)
- Verrucous Venous Malformation/ Hyperkeratotic Cutaneous Capillary-Venous Malformation (VVM/HCCVM) Genotype to phenotype: Dublin, Freiburg and Brussels HCPs involved (started 2016)
- Identification of novel genes for vascular anomalies: Dublin and Brussels HCPs involved
- VUS characterization using zebrafish: Freiburg and Brussels HCPs involved
- Year 1: Collaborative Publications
Blue Rubber Bleb Nevus (BRBN) Syndrome Is Caused by Somatic TEK (TIE2) Mutations.Soblet J, Kangas J, Nätynki M, Mendola A, Helaers R, Uebelhoer M, Kaakinen M, Cordisco M, Dompmartin A, Enjolras O, Holden S, Irvine AD, Kangesu L, Léauté-Labrèze C, Lanoel A, Lokmic Z, Maas S, McAleer MA, Penington A, Rieu P, Syed S, van der Vleuten C, Watson R, Fishman SJ, Mulliken JB, Eklund L, Limaye N, Boon LM, Vikkula M. J Invest Dermatol. 2017 Jan;137(1):207-216. doi: 10.1016/j.jid.2016.07.034. Epub 2016 Aug 9. PMID:27519652
Development of an international core outcome set for peripheral vascular malformations: the OVAMA project. Horbach SER, van der Horst CMAM, Blei F, van der Vleuten CJM, Frieden IJ, Richter GT, Tan ST, Muir T, Penington AJ, Boon LM, Spuls PI; OVAMA Consensus Group Br J Dermatol. 2017 Oct 7. doi: 10.1111/bjd.16029. [Epub ahead of print] PMID: 28986976
Germline Loss-of-Function Mutations in EPHB4 Cause a Second Form of Capillary Malformation-Arteriovenous Malformation (CM-AVM2) Deregulating RAS-MAPK Signaling. Amyere M, Revencu N, Helaers R, Pairet E, Baselga E, Cordisco M, Chung W, Dubois J, Lacour JP, Martorell L, Mazereeuw-Hautier J, Pyeritz RE, Amor DJ, Bisdorff A, Blei F, Bombei H, Dompmartin A, Brooks D, Dupont J, González-Enseñat MA, Frieden I, Gérard M, Kvarnung M, Hanson-Kahn AK, Hudgins L, Léauté-Labrèze C, McCuaig C, Metry D, Parent P, Paul C, Petit F, Phan A, Quere I, Salhi A, Turner A, Vabres P, Vicente A, Wargon O, Watanabe S, Weibel L, Wilson A, Willing M, Mulliken JB, Boon LM, Vikkula M. Circulation. 2017 Sep 12;136(11):1037-1048. doi: 10.1161/CIRCULATIONAHA.116.026886. Epub 2017 Jul 7. PMID: 28687708.
Blue Rubber Bleb Nevus (BRBN) Syndrome Is Caused by Somatic TEK (TIE2) Mutations. Soblet J, Kangas J, Nätynki M, Mendola A, Helaers R, Uebelhoer M, Kaakinen M, Cordisco M, Dompmartin A, Enjolras O, Holden S, Irvine AD, Kangesu L, Léauté-Labrèze C, Lanoel A, Lokmic Z, Maas S, McAleer MA, Penington A, Rieu P, Syed S, van der Vleuten C, Watson R, Fishman SJ, Mulliken JB, Eklund L, Limaye N, Boon LM, Vikkula M. J Invest Dermatol. 2017 Jan;137(1):207-216. doi: 10.1016/j.jid.2016.07.034. Epub 2016 Aug 9. PMID: 27519652.
- Year 2: Collaborative Publications
No collaborative publications from the VASCA-WG between March 2018-February 2019.
- Year 3-5: Collaborative Publications
* RASA1 mosaic mutations in patients with capillary malformation-arteriovenous malformation. Revencu N, Fastre E, Ravoet M, Helaers R, Brouillard P, Bisdorff-Bresson A, Chung CWT, Gerard M, Dvorakova V, Irvine AD, Boon LM, Vikkula M. J Med Genet. 2019 Jul 12. pii: jmedgenet-2019-106024. doi: 10.1136/jmedgenet-2019-106024. [Epub ahead of print] PMID:31300548
*A Clinical Feasibility Study To Image Angiogenesis in Patients With Arteriovenous Malformations Using 68Ga-RGD PET/CT. Lobeek D, Bouwman FCM, Aarntzen EHJG, Molkenboer-Kuenen JDM, Flucke UE, Nguyen HL, Vikkula M, Boon LM, Klein W, Laverman P, Oyen WJG, Boerman OC, Terry SYA, SchultzeKool LJ, Rijpkema M.J Nucl Med. 2019 Sep 13. pii: jnumed.119.231167. doi: 10.2967/jnumed.119.231167. [Epub ahead of print]
The Infantile Hemangioma Referral Score: A Validated Tool for Physicians. Léauté-Labrèze C, Baselga Torres E, Weibel L, Boon LM, El Hachem M, van der Vleuten C, Roessler J, Troilius Rubin A.Pediatrics. 2020 Apr;145(4):e20191628. doi: 10.1542/peds.2019-1628. Epub 2020 Mar 11.PMID: 32161112
New and Emerging Targeted Therapies for Vascular Malformations. Van Damme A, Seront E, Dekeuleneer V, Boon LM, Vikkula M. Am J Clin Dermatol. 2020 Oct;21(5):657-668. doi: 10.1007/s40257-020-00528-w. PMID: 32557381.
*Severe adverse events during sirolimus “off-label” therapy for vascular anomalies.Rössler J, Baselga E, Davila V, Celis V, Diociaiuti A, El Hachem M, Mestre S, Haeberli D, Prokop A, Hanke C, Loichinger W, Quéré I, Baumgartner I, Niemeyer CM, Kapp FG. Pediatr Blood Cancer. 2021 Aug;68(8):e28936. doi: 10.1002/pbc.28936. Epub 2021 Feb 13.PMID: 33580918
*Efficacy of Sirolimus in Patients Requiring Tracheostomy for Life-Threatening Lymphatic Malformation of the Head and Neck: A Report From the European Reference Network.
Holm A, Te Loo M, Schultze Kool L, Salminen P, Celis V, Baselga E, Duignan S, Dvorakova V, Irvine AD, Boon LM, Vikkula M, Ghaffarpour N, Niemeyer CM, Rössler J, Kapp FG.
Front Pediatr. 2021 Sep 30;9:697960. doi: 10.3389/fped.2021.697960. eCollection 2021.
PMID: 34660476
*Case report study of thalidomide therapy in 18 patients with severe arteriovenous malformations. Boon, L.M., Dekeuleneer, V., Coulie, Marot, L., Bataille, AC., Hammer, F., Clapuyt, P., Jeanjean, A., Dompmartin, A., Vikkula, M. Nat Cardiovasc Res 1, 562–567 (2022). https://doi.org/10.1038/s44161-022-00080-2
*The VASCERN-VASCA working group diagnostic and management pathways for severe and/or rare infantile hemangiomas. Diociaiuti A, Baselga E, Boon LM, Dompmartin A, Dvorakova V, El Hachem M, Gasparella P, Haxhija E, Ghaffarpour N, Kyrklund K, Irvine AD, Kapp FG, Rößler J, Salminen P, van den Bosch C, van der Vleuten C, Kool LS, Vikkula M. Eur J Med Genet. 2022 Jun;65(6):104517. doi: 10.1016/j.ejmg.2022.104517. Epub 2022 Apr 27. PMID: 35487416.
* VASCERN acknowledged